Unit of Rate Constant for Third Order Reaction
Calculation of rate constants of the processes of generation and relaxation of electronically and vibrationally excited particles are of significant importance. Rt kAn.
What Are The Units Of Rate Of Reaction In Chemistry Quora
If the units of time are s and of concentration are M then.

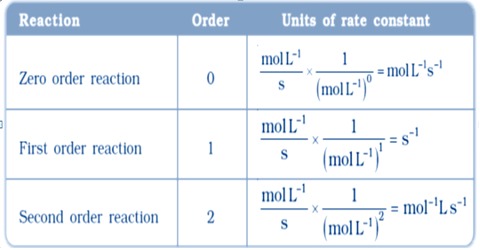
. For order two the rate constant has units of Lmol 1 s 1 or M 1 s 1 And for order three the rate constant has units of L 2 mol 2 s 1 or M 2 s 1 Plasma and gases. What are the units of the rate constant k for a second order reaction. Rt is the initial rate as a function of time t k is the rate constant A is the concentration of A and n is the order of A.
L2mol2 sec QUESTION 11 Given the data below for the reaction 2A 20-4CDE35 the reaction is order in A order in order in Cand order overall. QUESTION 10 Which of the following would be a reasonable unit for the rate constant of a third order reaction. Unit of K litre 2 mole -2 time-2.
Unit of rate constant k mol L-1s-1. General formulaUnit of rate constantmol lit-11-n sec-1So for third order reactionput n3please mark brainlist rockingtrishala3803 rockingtrishala3803 03022019. For a third order reaction the rate constant has units of liter squared per mole squares per second L 2 mol 2 s 1 or M 2 s 1 Other Calculations and Simulations For higher order reactions or for dynamic chemical reactions chemists apply a variety of molecular dynamics simulations using computer software.
In this reaction the rate will be written as. In a second-order reaction the. 5 rows In second example from the previous lesson a second-order reaction we found the units for k to.
M - n-1 s -1 etc. The unit of rate of reaction and rate of rate constant are same for a. Which one ofthem is the order.
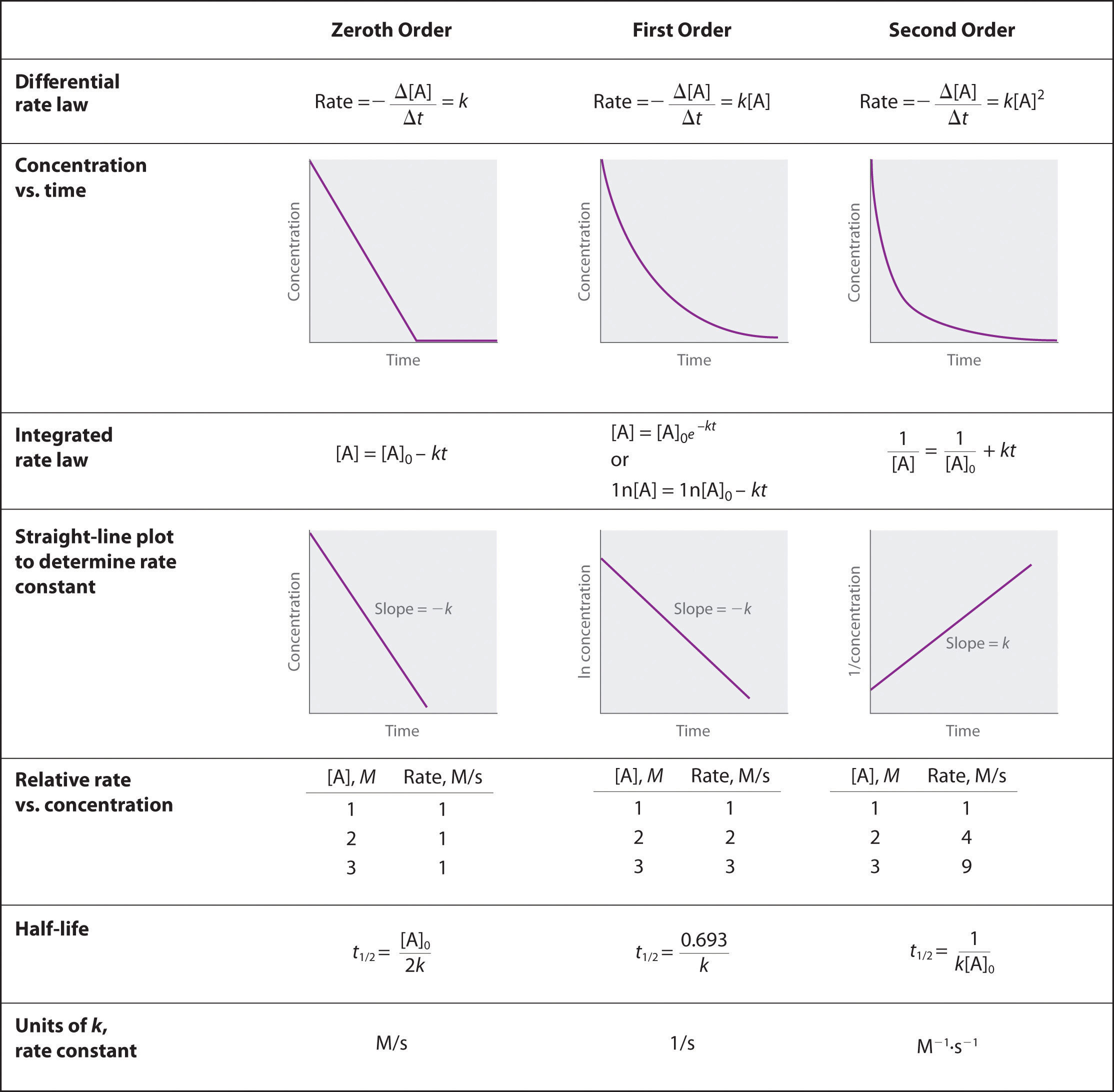
In this video you will learn the Units of rate constant for zero order first order second order third order and nth order reactionUnits of rate constant. The unit of rate constant for first order reaction is A. A zero-order reaction is a reaction having rate of concentration of reactant.
Click to see full answer. The unit of the rate constant in a zero-order reaction is given by concentrationtime or Ms where M is the molarity and s refers to one second. When a series of steps are involved in a reaction the overall rate of a reaction depends upon therates of.
In a chemical reaction the rate constant is independent of the initial concentration. R a t e d A d t k A 0 k. M -1 s -1 M -1 min -1 M -1 hr -1 etc.
2 The unit of velocity constant depends upon the units of concentration because. The rate of the reaction 2NOO 2 2NO 2 is given by RatekNO 2 O 2 Since it is a third-order reaction the unit of the rate constant is mol-2 s-1. Where k is a second order rate constant with units of M -1 min -1 or M -1 s -1.
Dimensional analysis requires the rate constant unit for a reaction whose overall order is x to be Lx-1mol1-xs-1. The units of k for a zero-order reaction are Ms the units of k for a first-order reaction are 1s and the units of k for a second. For order one the rate constant has units of s 1.
K is the rate constant of the reaction. Mt k M n. Two of the same reactant A combine in a single elementary step.
Find the rate constant unit for the reaction 2NOO 2 2NO 2 1 Mark Ans. R kA B Where k is known as the rate constant if the concentrations of both the reactants were one then the Rk which means that it wont depend on the initial concentration of the reactants. Second order reaction D.
For the third-order reaction described in the unit for k was derived to be L2mol-2s-1. Rate k A n. Since A is assumed the only reactant for simplicity its order IS the reaction order.
M -2 s -1 M -2 min -1 M -2 hr -1 etc. Characteristics of third order reaction 1 Unit of K-K 1 2t x 2a-x a 2a x 2 unit of K 1 time mole litre-1 mole litre-1 mole litre-1 2 x mole litre-12. TRA3 EU TRA3B LO TRA3B4 EK Transcript.
Rate k A 3 rate k A 2 B rate k A B C Mt k M 3. The rate refers to the rate of the reaction. The units of the rate constant k depend on the overall reaction order.
Therefore doubling the concentration of reactant A will quadruple the rate of the reaction.

Integrated Rate Law For A Third 3rd Order Reaction Youtube
No comments for "Unit of Rate Constant for Third Order Reaction"
Post a Comment